We all are familiar with the standard lithium ion batteries which are used in a variety of appliances in our daily life. The involve use of lithium compounds an as electrode. Lithium and similar elements used in such applications are not easily available and mining and extraction of lithium consumes a lot of energy which in turn adds to the carbon footprint of the battery manufacturing cycle. Also recycling lithium releases a lot of carbon dioxide into the air which in turn causes a lot of pollution.
Scientists at Rice University of New York and US Army Research Laboratory have designed a battery which uses Purpurin-a derivative of the madder root which is used in fabric dyeing. Madder roots have been used as a coloring agents since 3000 years and can provide orange, red and pink hues when used in different proportions
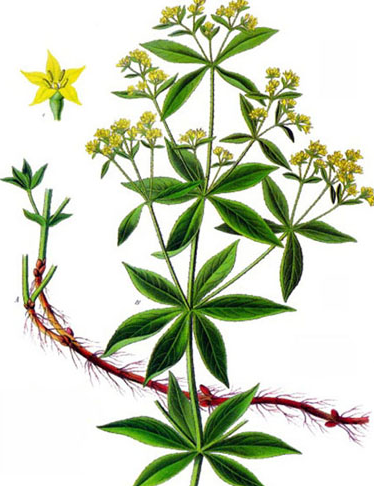
An extract of the dye-Purpurin is said to have properties similar to lithium electrodes such as carrying carbonyl and hydroxyl groups in the process of electrolysis. In standard batteries the electrodes are immersed in electrolytic solution and ions move from one electrode to the other creating a current. This current comes out of the electrode panels of the battery to run our appliances.
The core compound used here is chemically lithiatedPurpurin (CLP-where Purpurin is treated with lithium salts to create a material for the electrode) which is derived from the madder root. The project is being worked upon and it would take some more years for it to be made for commercial uses. However, it can for sure reduce our dependence on Lithium and cobalt metals for batteries and would also reduce the carbon footprint of the battery manufacturing cycle. Do watch out this space for more news on the Green Battery.